Page 57 - 11-chem-6
P. 57
6 CHEMICAL BONDING eLearn.Punjab
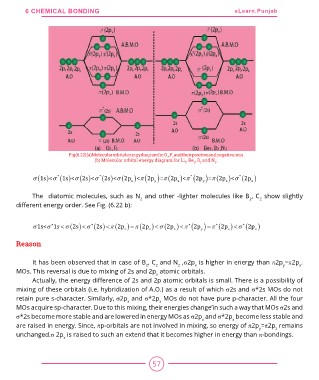
Fig(6.22)(a)MolecularorbitalenergydiagramforO2,F2andtheirpositiveandnegativeions
(b) Molecular orbital energy diagram.for Li2, Be2, B2 and N2.
s (1s)<s * (1s)<s (2s)<s * (2s)<s (2px )<p (2py )=p (2pz )<p * (2py )=p (2pz )<s * (2px )
The diatomic molecules, such as N2 and other -lighter molecules like B2, C2 show slightly
different energy order. See Fig. (6.22 b):
( ) s 1s<s *1s < s (2s) < s * (2s) < p (2pz ) = p (2pz ) < s (2px ) < p * 2py = p * (2pz ) < s * (2px )
Reason
It has been observed that in case of B2, C2 and N2 ,s2px is higher in energy than p2py=p2px.
MOs. This reversal is due to mixing of 2s and 2px atomic orbitals.
Actually, the energy difference of 2s and 2p atomic orbitals is small. There is a possibility of
mixing of these orbitals (i.e. hybridization of A.O.) as a result of which s2s and s*2s MOs do not
retain pure s-character. Similarly, s2px and s*2px MOs do not have pure p-character. All the four
MOs acquire sp-character. Due to this mixing, their energies change’in such a way that MOs s2s and
s*2s become more stable and are lowered in energy MOs as s2px and s*2px become less stable and
are raised in energy. Since, pp-orbitals are not involved in mixing, so energy of p2py=p2pz remains
unchanged.s 2px is raised to such an extend that it becomes higher in energy than p-bondings.
57